Background
In the setting of high tumor burden, acute kidney injury (AKI), plasma cell leukemia, and extramedullary disease (EMD), rapid control of MM can be required. Our group has previously published data with HyperCy-Bortezomib-Doxorubicin-Dex (Tabchi et al. J Leuk Lymphoma 2019) and Daratumumab (Dara)-HyperCy-Dex +/- Car (Shank et al. ASCO 2020).
When limiting toxicities from other agents is a priority or baseline end-organ damage limits the use of other drugs, HyperCy-Dex alone has also been used at our center to effect rapid disease response. For some patients, Car has been combined with HyperCy-Dex to add another mechanism of myeloma cell-killing. We here report response and safety outcomes with HyperCy-Dex +/- Car in this retrospective analysis of pts with newly diagnosed (ND) and relapsed/refractory (RR) MM.
Methods
Between 5/2016 and 8/2019, 53 pts received at least 1 cycle (28 days) of HyperCy (300-350 mg/m2 IV q12h x 6-8 doses)-Dex +/- Car (20-36 mg/m2). Cyclophosphamide was not dose adjusted for renal or hepatic impairment per institutional practice. Response was assessed by International Myeloma Working Group Uniform Criteria. High risk cytogenetics were defined as the presence of 17p deletion, t(14;16), and/or t(4;14). Overall response rate (ORR) and disease control rate (DCR) were defined as ≥ partial response and ≥ stable disease, respectively. Patients were censored for progression free survival (PFS) at the last follow-up date if neither progression nor death occurred. All pts received growth factors, and viral, bacterial, fungal, and peptic ulcer prophylaxis.
Results
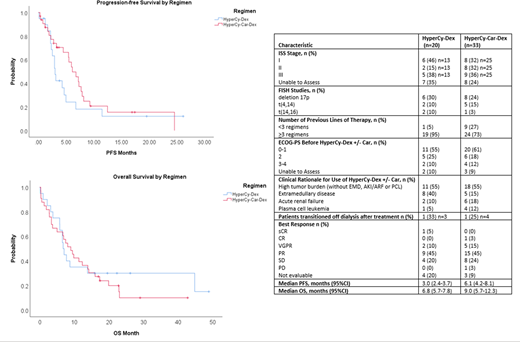
The median age (years) of patients receiving HyperCy-Dex (n=20) and HyperCy-Car-Dex (n=33) was 56 (38-77) and 58 (36-79), respectively. Respective frequencies of male gender (40% vs 39%), ISS stage III (38% vs 36%), baseline ECOG 3-4 (10% vs 12%), high risk cytogenetics (40% vs 36%) and previously untreated disease (5% vs 6%) with HyperCy-Dex and HyperCy-Car-Dex were similar. Median number (no.) of prior therapies was higher in the HyperCy-Dex group [n = 6.5 (range 1 -15)] compared with the HyperCy-Car-Dex group [n = 4 (range 0-11)]. Most patients had been previously exposed to bortezomib (90% vs 79%), lenalidomide (95% vs 79%), pomalidomide (60% vs 58%), Dara (75% vs 64%), and Car (85% vs 76%). Many patients had received >2 immunomodulators (IMiDs) (60% vs 61%), >2 proteasome inhibitors (75% vs 61%), and/or an IMiD, PI, and Dara (75% vs 64%). Most had also undergone prior autologous stem cell transplantation (ASCT) (80% vs 70%).
The most common indication for HyperCy-Dex +/- Car was high tumor burden (both cohorts 55%), followed by EMD (40% vs 15%), and AKI (10% vs 18%). In each cohort, the median no. of cycles administered was 1 [range (1-4) for HyperCy-Dex and (1-3) for HyperCy-Car-Dex]. Rates of best response were ≥ complete response (CR) (5% vs 3%), very good partial response (VGPR) (10% vs 15%), partial response (PR) (45% vs 45%), and stable disease (SD) (20% vs 24%). ORRs were 60% vs 64%, with DCRs of 80% vs 88%. Median PFS (months) was 3 (HyperCy-Dex) vs 6.1 (HyperCy-Car-Dex), and median overall survival (months) was 6.8 vs 9, respectively. One pt (5%) in the HyperCy-Dex group and 4 pts (12%) in the HyperCy-Car-Dex group were bridged to ASCT within 60 days of treatment. Of 2 pts treated with HyperCy-Dex due to AKI, the 1 evaluable pt had a serum creatinine (SCr) improvement of 16.4%. Among 6 evaluable pts treated with HyperCy-Car-Dex, the median SCr improvement was 55%.
Grade 3-4 toxicities included thrombocytopenia (70% vs 91%), anemia (65% vs 85%), neutropenia (50% vs 73%), and febrile neutropenia (FN) (30% vs 55%). Other toxicities (all grades) included pneumonia (25% vs 36%), viral infections (30% vs 24%), sepsis (15% vs 6%), and congestive heart failure (0% vs 3%). Re-hospitalization within 28 days of treatment was required for 65% and 79% of patients, respectively. Rates of treatment related mortality were 5% and 9%, respectively.
Conclusions
HyperCy-Dex +/- Car is effective in achieving rapid disease control in the setting of aggressive disease, with high rates of ORR and DCR allowing bridging to ASCT or investigational chimeric antigen receptor T cell therapy, improvement of EMD, and/or renal recovery for many patients. However, rates of hematologic toxicity and infections are high, warranting twice weekly monitoring for transfusion needs and early detection of other toxicities.
Kaufman:Janssen: Research Funding; Karyopharm: Honoraria; Bristol Myers Squibb: Research Funding. Lee:Celgene: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Daiichi Sankyo: Research Funding; Regeneron: Research Funding; Genentech: Consultancy; Genentech: Consultancy; Takeda: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Manasanch:Quest Diagnostics: Research Funding; Merck: Research Funding; JW Pharma: Research Funding; Glaxo Smith Kline: Honoraria; Adaptive Biotechnologies: Honoraria; BMS: Honoraria; Takeda: Honoraria; Sanofi: Honoraria, Research Funding; Novartis: Research Funding. Patel:Nektar: Consultancy, Research Funding; Cellectis: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Oncopeptides: Consultancy; Poseida: Research Funding; Janssen: Consultancy, Research Funding; Precision Biosciences: Research Funding; Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding. Orlowski:Amgen, Inc., AstraZeneca, BMS, Celgene, EcoR1 Capital LLC, Forma Therapeutics, Genzyme, GSK Biologicals, Ionis Pharmaceuticals, Inc., Janssen Biotech, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Regeneron Pharmaceuticals, Inc.,: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Aventis, Servier, Takeda Pharmaceuticals North America, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; STATinMED Research: Consultancy; Founder of Asylia Therapeutics, Inc., with associated patents and an equity interest, though this technology does not bear on the current submission.: Current equity holder in private company, Patents & Royalties; Laboratory research funding from BioTheryX, and clinical research funding from CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding. Thomas:Xencor: Research Funding; X4 Pharma: Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Pharmacyclics: Other: Advisory Boards; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal